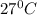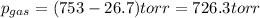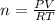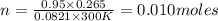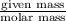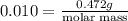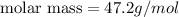Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
10. in an experiment in a general chemistry laboratory, a student collected a sample of a gas over w...
Questions

Social Studies, 05.04.2021 17:00


History, 05.04.2021 17:00

Mathematics, 05.04.2021 17:00


Mathematics, 05.04.2021 17:00


English, 05.04.2021 17:00

Social Studies, 05.04.2021 17:00


History, 05.04.2021 17:00

Mathematics, 05.04.2021 17:00





Mathematics, 05.04.2021 17:00



History, 05.04.2021 17:00

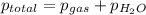
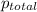 =753 torr
=753 torr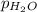 = 26.7 torr at
= 26.7 torr at