Chemistry, 13.07.2019 23:20 roxymiller3942
The standard cell potential ec for the reduction of silver ions with elemental copper is 0.46v at 25 degrees celsius. calculate δg for this reaction.
*** explain the reactions since i’m very confused as to wich side i should put the electrons.
ex: cu-> cu2+ + 2e

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2


Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
The standard cell potential ec for the reduction of silver ions with elemental copper is 0.46v at 25...
Questions

Mathematics, 27.01.2021 02:00

Mathematics, 27.01.2021 02:00

English, 27.01.2021 02:00

Mathematics, 27.01.2021 02:00



Mathematics, 27.01.2021 02:00


English, 27.01.2021 02:00



Mathematics, 27.01.2021 02:00

History, 27.01.2021 02:00



Mathematics, 27.01.2021 02:00

Mathematics, 27.01.2021 02:00

Chemistry, 27.01.2021 02:00


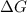 for this reaction is, -88780 J/mole.
for this reaction is, -88780 J/mole.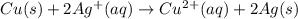
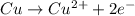
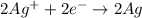
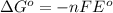
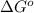 = Gibbs free energy = ?
= Gibbs free energy = ?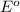 = standard e.m.f of cell = 0.46 V
= standard e.m.f of cell = 0.46 V