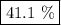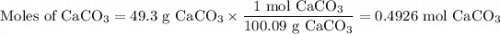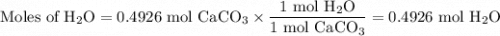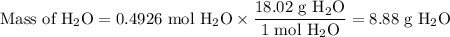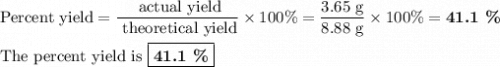Chemistry, 15.07.2019 02:10 raymond5799
A49.3 sample of caco3 was treated with aqueous h2so4, producing calcium sulfate, 3.65 g of water and co2(g). what was the % yield of h2o?

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 21.06.2019 19:10
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3
You know the right answer?
A49.3 sample of caco3 was treated with aqueous h2so4, producing calcium sulfate, 3.65 g of water and...
Questions


Mathematics, 14.01.2021 06:50

Physics, 14.01.2021 06:50



Biology, 14.01.2021 06:50



Mathematics, 14.01.2021 06:50


Mathematics, 14.01.2021 06:50


Mathematics, 14.01.2021 06:50

Chemistry, 14.01.2021 06:50


English, 14.01.2021 06:50


Chemistry, 14.01.2021 06:50

Mathematics, 14.01.2021 06:50