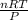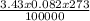Chemistry, 15.07.2019 17:20 pamelperezz26
How many liters of radon gas would be in 3.43 moles at standard temperature and pressure (273k and 100 kpa)?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
You know the right answer?
How many liters of radon gas would be in 3.43 moles at standard temperature and pressure (273k and 1...
Questions

English, 30.11.2021 14:00

Mathematics, 30.11.2021 14:00

English, 30.11.2021 14:00



English, 30.11.2021 14:00

English, 30.11.2021 14:00

English, 30.11.2021 14:00

Mathematics, 30.11.2021 14:00


Biology, 30.11.2021 14:00

English, 30.11.2021 14:00

Mathematics, 30.11.2021 14:00

Mathematics, 30.11.2021 14:00

English, 30.11.2021 14:00

SAT, 30.11.2021 14:00

Mathematics, 30.11.2021 14:00

English, 30.11.2021 14:00

English, 30.11.2021 14:00

Mathematics, 30.11.2021 14:00