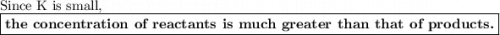Chemistry, 16.07.2019 03:10 19dansiste
The equilibrium-constant of the reaction no2(g)+no3(g)⇌n2o5(g) is k=2.1×10−20. what can be said about this reaction? a. at equilibrium the concentration of products and reactants is about the same. b. at equilibrium the concentration of products is much greater than the concentration of reactants. c. at equilibrium the concentration of reactants is much greater than that of products. d. there are no reactants left over once the reaction reaches equilibrium.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2

Chemistry, 23.06.2019 10:30
An atom that gains or loses one or more electrons is called a(n)
Answers: 1
You know the right answer?
The equilibrium-constant of the reaction no2(g)+no3(g)⇌n2o5(g) is k=2.1×10−20. what can be said abou...
Questions

Biology, 06.11.2020 04:40


English, 06.11.2020 04:40

English, 06.11.2020 04:40

Mathematics, 06.11.2020 04:40


History, 06.11.2020 04:40

Social Studies, 06.11.2020 04:40




Chemistry, 06.11.2020 04:40


History, 06.11.2020 04:40


Biology, 06.11.2020 04:40

English, 06.11.2020 04:40


Mathematics, 06.11.2020 04:40

![K = \dfrac{[\text{Products}]}{[\text{Reactants}]}](/tpl/images/0094/8248/37418.png)