
Chemistry, 16.07.2019 12:10 kinziemadison12
Asample of cesium carbonate, weighing 3.80 g, requires 1.90 g of hydrogen bromide gas to completely decompose to water, cesium bromide, and carbon dioxide gas. the total mass of water and cesium bromide formed is 5.20 g and no hydrogen bromide or cesium carbonate remains. according to the law of conservation of mass, what mass of carbon dioxide must have been formed?
a. 0.50 g
b 1.40 g
c 5.49 g
d 10.90 g
e 1.90 g
give me an explanation on why it is the correct answer.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
The length of a vector arrow represents its magnitude and the point represents its direction true or false apex
Answers: 3

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2
You know the right answer?
Asample of cesium carbonate, weighing 3.80 g, requires 1.90 g of hydrogen bromide gas to completely...
Questions


Biology, 17.03.2022 09:50

Computers and Technology, 17.03.2022 09:50

Mathematics, 17.03.2022 09:50

SAT, 17.03.2022 09:50

Mathematics, 17.03.2022 09:50

SAT, 17.03.2022 09:50

Chemistry, 17.03.2022 09:50


Chemistry, 17.03.2022 14:00

Mathematics, 17.03.2022 14:00

English, 17.03.2022 14:00

Mathematics, 17.03.2022 14:00

Mathematics, 17.03.2022 14:00



Computers and Technology, 17.03.2022 14:00

Spanish, 17.03.2022 14:00


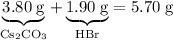 .
.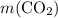 represent the mass of carbon dioxide produced in this reaction.
represent the mass of carbon dioxide produced in this reaction.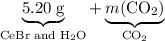 .
.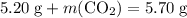 .
.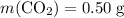 .
.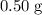 .
.


