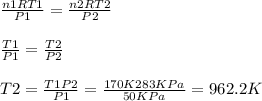Chemistry, 16.07.2019 21:40 frankgore7836
Acontainer of carbon dioxide (co2) has an initial temperature of 170 k with a pressure of 50 kpa. when the container is heated the pressure is measured at 283 kpa. the volume is constant at 12 l throughout. what is the final temperature of the carbon dioxide (co2)?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Acontainer of carbon dioxide (co2) has an initial temperature of 170 k with a pressure of 50 kpa. wh...
Questions

Chemistry, 26.01.2021 03:10




Physics, 26.01.2021 03:10

Mathematics, 26.01.2021 03:10

Mathematics, 26.01.2021 03:10

Mathematics, 26.01.2021 03:10


Arts, 26.01.2021 03:10

Mathematics, 26.01.2021 03:10


Mathematics, 26.01.2021 03:10



Mathematics, 26.01.2021 03:10



French, 26.01.2021 03:10