Which statement best describes balancing equations and the law of conservation of mass?
there...

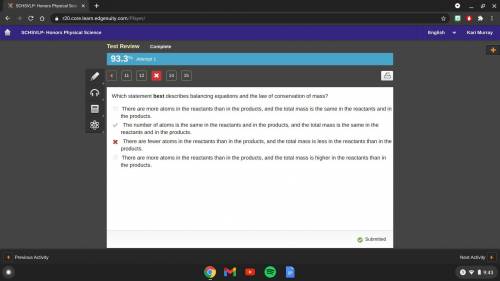
Which statement best describes balancing equations and the law of conservation of mass?
there are more atoms in the reactants than in the products, and the total mass is the same in the reactants and in the
products
the number of atoms is the same in the reactants and in the products, and the total mass is the same in the reactants and
in the products
there are fewer atoms in the reactants than in the products, and the total mass is less in the reactants than in the
products
there are more atoms in the reactants than in the products, and the total mass is higher in the reactants than in the
products

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1


Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
Questions

Chemistry, 23.10.2021 20:30


Mathematics, 23.10.2021 20:30







Geography, 23.10.2021 20:30




Mathematics, 23.10.2021 20:30


Mathematics, 23.10.2021 20:30


Computers and Technology, 23.10.2021 20:30

Biology, 23.10.2021 20:30

Physics, 23.10.2021 20:30