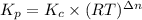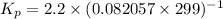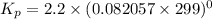Chemistry, 18.07.2019 21:10 GhostElite295
Calculate the equilibrium constant kp for this reaction, given the following information (at 299 k ): 2no(g)+br2(g)⇌2nobr(g)kc=2.2 2no(g)⇌n2(g)+o2(g)kc=2.3×1030

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 23.06.2019 06:40
4786 joules of heat are transferred to a 89.0 gramsample of an unknown material, with an initialtemperature of 23.0°c. what is the specific heat of thematerialif the final temperature is 89.5 °c?
Answers: 1
You know the right answer?
Calculate the equilibrium constant kp for this reaction, given the following information (at 299 k )...
Questions

Mathematics, 14.07.2019 10:50

Mathematics, 14.07.2019 10:50







Mathematics, 14.07.2019 10:50

Mathematics, 14.07.2019 10:50

Mathematics, 14.07.2019 10:50

Mathematics, 14.07.2019 10:50

History, 14.07.2019 10:50


Mathematics, 14.07.2019 10:50

Mathematics, 14.07.2019 10:50


Health, 14.07.2019 10:50


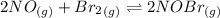 , Kp = 0.08967
, Kp = 0.08967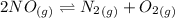 , Kp = 2.3×10³⁰
, Kp = 2.3×10³⁰