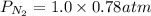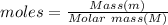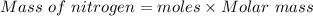Chemistry, 18.07.2019 22:10 legendman27
Calculate the mass of nitrogen dissolved at room temperature in an 86.0 l home aquarium. assume a total pressure of 1.0 atm and a mole fraction for nitrogen of 0.78.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

You know the right answer?
Calculate the mass of nitrogen dissolved at room temperature in an 86.0 l home aquarium. assume a to...
Questions

Mathematics, 27.09.2019 01:00













Computers and Technology, 27.09.2019 01:00






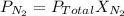
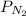 is the partial pressure of nitrogen
is the partial pressure of nitrogen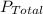 is the Total pressure
is the Total pressure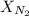 is the mole fraction of nitrogen
is the mole fraction of nitrogen