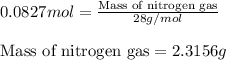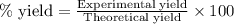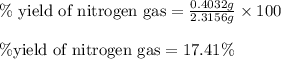Chemistry, 18.07.2019 22:10 jgstyle2388
Hydrazine, n2h4 , reacts with oxygen to form nitrogen gas and water. n2h4(aq)+o2(g)⟶n2(g)+2h2o(l) if 2.65 g of n2h4 reacts with excess oxygen and produces 0.350 l of n2 , at 295 k and 1.00 atm, what is the percent yield of the reaction?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1

Chemistry, 23.06.2019 10:00
How to draw a diagram to represent a calcium metal lattice?
Answers: 3
You know the right answer?
Hydrazine, n2h4 , reacts with oxygen to form nitrogen gas and water. n2h4(aq)+o2(g)⟶n2(g)+2h2o(l) if...
Questions


Mathematics, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30



Mathematics, 18.03.2021 03:30

Geography, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30



Mathematics, 18.03.2021 03:30


Spanish, 18.03.2021 03:30


English, 18.03.2021 03:30

Health, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30


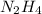
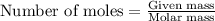
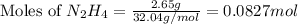
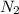
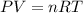
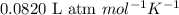
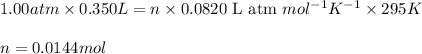
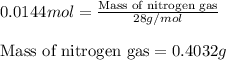
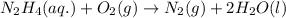
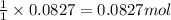 of nitrogen gas.
of nitrogen gas.