

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1


Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
What is the limiting reactant when 1.50 g of lithium and 1.50 g of nitrogen combine to form lithium...
Questions

English, 09.03.2021 20:40

Mathematics, 09.03.2021 20:40


History, 09.03.2021 20:40



Mathematics, 09.03.2021 20:40


Mathematics, 09.03.2021 20:40

English, 09.03.2021 20:40

Mathematics, 09.03.2021 20:40



History, 09.03.2021 20:40

Mathematics, 09.03.2021 20:40


English, 09.03.2021 20:40



Mathematics, 09.03.2021 20:40

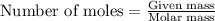 ....(1)
....(1)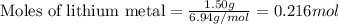
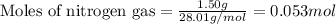
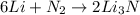
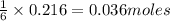 of nitrogen gas.
of nitrogen gas.

