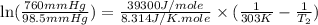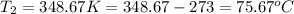Chemistry, 19.07.2019 00:10 melanie7152
The vapor pressure of ethanol is 30°c at 98.5 mmhg and the heat of vaporization is 39.3 kj/mol. determine the normal boiling point of ethanol from this data.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1


Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
The vapor pressure of ethanol is 30°c at 98.5 mmhg and the heat of vaporization is 39.3 kj/mol. dete...
Questions



Mathematics, 17.12.2020 02:50








Chemistry, 17.12.2020 02:50

Mathematics, 17.12.2020 02:50



Mathematics, 17.12.2020 02:50



Biology, 17.12.2020 02:50

Mathematics, 17.12.2020 02:50

 or
or 
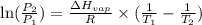
 = vapor pressure of ethanol at
= vapor pressure of ethanol at 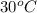 = 98.5 mmHg
= 98.5 mmHg = vapor pressure of ethanol at normal boiling point = 1 atm = 760 mmHg
= vapor pressure of ethanol at normal boiling point = 1 atm = 760 mmHg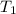 = temperature of ethanol =
= temperature of ethanol = 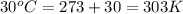
 = normal boiling point of ethanol = ?
= normal boiling point of ethanol = ?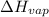 = heat of vaporization = 39.3 kJ/mole = 39300 J/mole
= heat of vaporization = 39.3 kJ/mole = 39300 J/mole