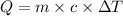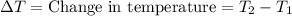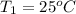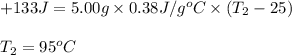Chemistry, 19.07.2019 01:20 katelynn73
A5.00-g sample of copper metal at 25.0 °c is heated by the addition of 133 j of energy. the final temperature of the copper is °c. the specific heat capacity of copper is 0.38 j/g°c.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1
You know the right answer?
A5.00-g sample of copper metal at 25.0 °c is heated by the addition of 133 j of energy. the final te...
Questions

Mathematics, 20.08.2019 07:30

Mathematics, 20.08.2019 07:30

Mathematics, 20.08.2019 07:30







History, 20.08.2019 07:30

Mathematics, 20.08.2019 07:30

Mathematics, 20.08.2019 07:30

Mathematics, 20.08.2019 07:30


English, 20.08.2019 07:30

Mathematics, 20.08.2019 07:30