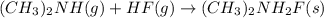Chemistry, 19.07.2019 03:20 Brookwiggington8814
Using the lewis concept of acids and bases, identify the lewis acid and base in each of the following reactions: ni(no3)3(s) 6h2o(l)→ni(h2o)63 (aq) 3no3−(aq) (ch3)2nh(g) hf(g)→(ch3)2nh2f(s)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
You know the right answer?
Using the lewis concept of acids and bases, identify the lewis acid and base in each of the followin...
Questions

History, 21.02.2020 03:27

Mathematics, 21.02.2020 03:27



Business, 21.02.2020 03:27


History, 21.02.2020 03:27





Computers and Technology, 21.02.2020 03:28



Social Studies, 21.02.2020 03:28

Mathematics, 21.02.2020 03:28



SAT, 21.02.2020 03:28

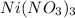 and Lewis-base =
and Lewis-base = 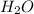
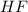 and Lewis-base =
and Lewis-base = 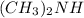 [/tex]
[/tex]![Ni(NO_3)_3(s)+6H_2O(l)\rightarrow [Ni(H_2O)_6]^{3+}(aq)+3NO_3^-(aq)](/tpl/images/0106/3688/a6dfb.png)
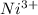 which is a Lewis-acid.
which is a Lewis-acid.