
Chemistry, 19.07.2019 03:30 Wolfgirl2032
Which of the following assumptions appears reasonable for the isothermal process? h20 (liq, 1 bar) → h2o (liq, 1300 bar), t = 20°c. a. au = 0, ah 0 b. au = 0, ah 0 c. au #0, ah = 0 d. none of the above hinn nf diethyl ether using the chen's rule is

Answers: 1
Another question on Chemistry



Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
Which of the following assumptions appears reasonable for the isothermal process? h20 (liq, 1 bar)...
Questions

History, 10.05.2021 15:40

Mathematics, 10.05.2021 15:40

Physics, 10.05.2021 15:40

Mathematics, 10.05.2021 15:40




Mathematics, 10.05.2021 15:40

Social Studies, 10.05.2021 15:40

Mathematics, 10.05.2021 15:40


Mathematics, 10.05.2021 15:50

Business, 10.05.2021 15:50

Mathematics, 10.05.2021 15:50

Mathematics, 10.05.2021 15:50

Chemistry, 10.05.2021 15:50

Health, 10.05.2021 15:50


Mathematics, 10.05.2021 15:50

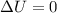 and
and 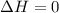
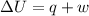
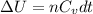
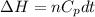
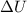 = internal energy
= internal energy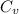 = specific heat capacity at constant volume
= specific heat capacity at constant volume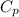 = specific heat capacity at constant pressure
= specific heat capacity at constant pressure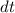 = change in temperature
= change in temperature = 0
= 0

