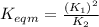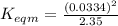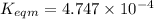Chemistry, 19.07.2019 04:30 yarielisr18
Use the reactions below and their equilibrium constants to predict the equilibrium constant for the reaction 2a(s)⇌3d(g). a(s) ⇌ 12 b(g)+c(g), k1=0.0334 3d(g) ⇌ b(g)+2c(g), k2=2.35

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3

Chemistry, 23.06.2019 09:20
1) a. water molecule breaks up into hydrogen and oxygen on passing electricity. does this involve breaking intermolecular or intramolecular forces of attraction. explain b. on boiling water changes to water vapor. does this involve breaking intermolecular or intramolecular forces of attraction. explain methanol evaporates faster than water. contrast the intermolecular forces and the vapor pressures of methanol and water?
Answers: 2
You know the right answer?
Use the reactions below and their equilibrium constants to predict the equilibrium constant for the...
Questions


Physics, 02.02.2020 09:42

Advanced Placement (AP), 02.02.2020 09:42


Mathematics, 02.02.2020 09:42

English, 02.02.2020 09:42


Mathematics, 02.02.2020 09:42










Mathematics, 02.02.2020 09:42


Mathematics, 02.02.2020 09:42


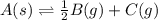
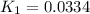
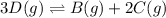

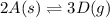
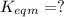
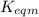 for the final reaction.
for the final reaction.