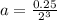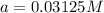Chemistry, 19.07.2019 04:30 mstahuggoo6102
For the first-order reaction, 2 n2o(g) → 2 n2(g) + o2(g), what is the concentration of n2o after 3 half-lives if 0.25 mol of n2o is initially placed into a 1.00-l reaction vessel?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
For the first-order reaction, 2 n2o(g) → 2 n2(g) + o2(g), what is the concentration of n2o after 3 h...
Questions



Mathematics, 02.10.2019 14:50


Mathematics, 02.10.2019 14:50

History, 02.10.2019 14:50

Arts, 02.10.2019 14:50



History, 02.10.2019 14:50


Computers and Technology, 02.10.2019 15:00

English, 02.10.2019 15:00


Mathematics, 02.10.2019 15:00



History, 02.10.2019 15:00

Mathematics, 02.10.2019 15:00

Physics, 02.10.2019 15:00

 after 3 half-lives is 0.03125 M.
after 3 half-lives is 0.03125 M.
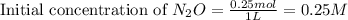
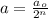
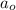 = Initial concentration of the reactant = 0.25 mol/L
= Initial concentration of the reactant = 0.25 mol/L