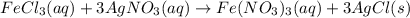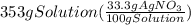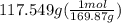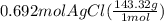Chemistry, 19.07.2019 20:10 laurielaparr2930
300. ml of a 3.0 m aqueous solution of iron (iii) chloride is mixed with 353 grams of a 33.3 mass % solution of silver (u) nitrate and water. 267 grams of a solid precipitate forms. what is the percent yield of the reaction assuming that the solubility of the solid precipitate in water is negligible.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
300. ml of a 3.0 m aqueous solution of iron (iii) chloride is mixed with 353 grams of a 33.3 mass %...
Questions




History, 16.11.2020 22:30

Business, 16.11.2020 22:30

Chemistry, 16.11.2020 22:30

Biology, 16.11.2020 22:30

English, 16.11.2020 22:30


Chemistry, 16.11.2020 22:30


English, 16.11.2020 22:30


Mathematics, 16.11.2020 22:30


Chemistry, 16.11.2020 22:30

Chemistry, 16.11.2020 22:30


Health, 16.11.2020 22:30

Mathematics, 16.11.2020 22:30