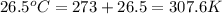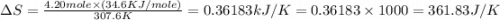Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

You know the right answer?
Calculate the change in entropy that occurs in the system when 4.20 mole of diethyl ether (\(\rm c_4...
Questions





Mathematics, 29.11.2019 00:31

Mathematics, 29.11.2019 00:31

Biology, 29.11.2019 00:31


Mathematics, 29.11.2019 00:31

Mathematics, 29.11.2019 00:31

Biology, 29.11.2019 00:31

Mathematics, 29.11.2019 00:31

Geography, 29.11.2019 00:31


Mathematics, 29.11.2019 00:31

English, 29.11.2019 00:31


Mathematics, 29.11.2019 00:31


Mathematics, 29.11.2019 00:31

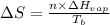
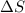 = entropy change of the system = ?
= entropy change of the system = ?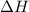 = enthalpy of vaporization = 34.6 kJ/mole
= enthalpy of vaporization = 34.6 kJ/mole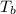 = normal boiling point =
= normal boiling point =