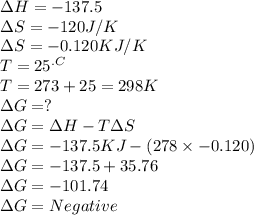Chemistry, 19.07.2019 22:20 haleybain6353
C2h4(g) + h2(g) → c2h6(g) δh = –137.5 kj; δs = –120.5 j/k calculate δg at 25 °c and determine whether the reaction is spontaneous. does δg become more negative or more positive as the temperature increases?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1


Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
C2h4(g) + h2(g) → c2h6(g) δh = –137.5 kj; δs = –120.5 j/k calculate δg at 25 °c and determine wheth...
Questions

Mathematics, 22.01.2021 14:40

Biology, 22.01.2021 14:40

Mathematics, 22.01.2021 14:40



Biology, 22.01.2021 14:40




Mathematics, 22.01.2021 14:40


Mathematics, 22.01.2021 14:50


Mathematics, 22.01.2021 14:50

Mathematics, 22.01.2021 14:50

Physics, 22.01.2021 14:50

Physics, 22.01.2021 14:50


Mathematics, 22.01.2021 14:50

Mathematics, 22.01.2021 14:50