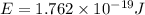Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
Calculate the energy for vacancy formation in silver, given that the equilibrium number of vacancies...
Questions

Mathematics, 09.10.2021 02:00


Advanced Placement (AP), 09.10.2021 02:00

Mathematics, 09.10.2021 02:00


Mathematics, 09.10.2021 02:00


Biology, 09.10.2021 02:00



English, 09.10.2021 02:00

Computers and Technology, 09.10.2021 02:00



English, 09.10.2021 02:00



Mathematics, 09.10.2021 02:00

Mathematics, 09.10.2021 02:00

Mathematics, 09.10.2021 02:00

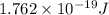
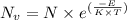
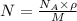
![N_v=[\frac{N_A\times \rho}{M}]\times e^{(\frac{-E}{K\times T})}](/tpl/images/0109/4101/22c5a.png)
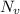 = equilibrium number of vacancies =
= equilibrium number of vacancies = 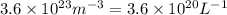
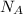 = Avogadro's number =
= Avogadro's number = 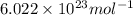
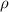 = density =
= density = 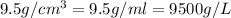
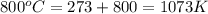
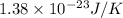
![3.6\times 10^{20}L^{-1}=[\frac{(6.022\times 10^{23}mol^{-1})\times 9500g/L}{107.9g/mol}]\times e^{[\frac{-E}{(1.38\times 10^{-23}J/K)\times 1073K}]}](/tpl/images/0109/4101/c481f.png)