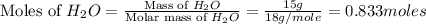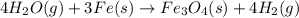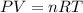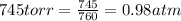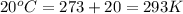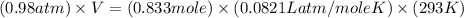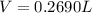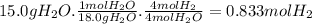Cavendish prepared hydrogen in 1766 by the novel method of passing steam through a red-hot gun barrel: 4h2 o(g) + 3fe(s) ⟶ fe3 o4 (s) + 4h2 (g) (a) outline the steps necessary to answer the following question: what volume of h2 at a pressure of 745 torr and a temperature of 20 °c can be prepared from the reaction of 15.o g of h2o? (b) answer the question.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1


Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

You know the right answer?
Cavendish prepared hydrogen in 1766 by the novel method of passing steam through a red-hot gun barre...
Questions

Mathematics, 12.02.2020 22:30





Mathematics, 12.02.2020 22:30


Mathematics, 12.02.2020 22:30


Geography, 12.02.2020 22:30

Mathematics, 12.02.2020 22:30

Biology, 12.02.2020 22:30

Mathematics, 12.02.2020 22:30


Advanced Placement (AP), 12.02.2020 22:44


Physics, 12.02.2020 22:45

Mathematics, 12.02.2020 22:45


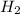 will be, 0.2690 L
will be, 0.2690 L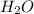 .
.