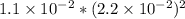Chemistry, 19.07.2019 23:20 jasmine3051
Excess ca(oh)2 is shaken with water to produce a saturated solution. the solution is filtered, and a 50.00 ml sample titrated with hcl requires 11.22 ml of 0.0983 m hcl to reach the end point. part a calculate ksp for ca(oh)2.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3
You know the right answer?
Excess ca(oh)2 is shaken with water to produce a saturated solution. the solution is filtered, and a...
Questions


Mathematics, 27.10.2020 09:10


Biology, 27.10.2020 09:10




Business, 27.10.2020 09:10

Mathematics, 27.10.2020 09:10

History, 27.10.2020 09:10

Mathematics, 27.10.2020 09:10

English, 27.10.2020 09:10

Physics, 27.10.2020 09:10


History, 27.10.2020 09:10

Mathematics, 27.10.2020 09:10

Physics, 27.10.2020 09:10

Physics, 27.10.2020 09:10

English, 27.10.2020 09:10

Mathematics, 27.10.2020 09:10

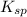 for calcium hydroxide is
for calcium hydroxide is 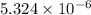
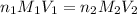
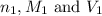 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 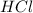
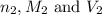 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is 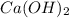
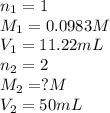
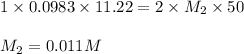
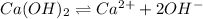
![K_{sp}=[Ca^{2+}][OH^-]^2](/tpl/images/0109/5708/8de55.png)
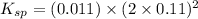
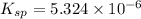
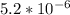
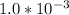
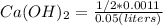
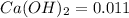 .
.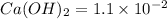 .
.