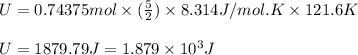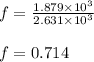Chemistry, 20.07.2019 02:30 aubriebv2020
Suppose 23.8 g of oxygen (o2) is heated at constant atmospheric pressure from 27.4°c to 149°c. (a) how many moles of oxygen are present? (take the molar mass of oxygen to be 32.0 g/mol) (b) how much energy is transferred to the oxygen as heat? (the molecules rotate but do not oscillate.) (c) what fraction of the heat is used to raise the internal energy of the oxygen?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1
You know the right answer?
Suppose 23.8 g of oxygen (o2) is heated at constant atmospheric pressure from 27.4°c to 149°c. (a) h...
Questions

Mathematics, 30.11.2021 01:00










Mathematics, 30.11.2021 01:00









SAT, 30.11.2021 01:00

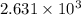
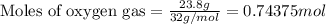
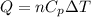
 = specific heat capacity at constant pressure =
= specific heat capacity at constant pressure = 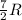 (For diatomic gas)
(For diatomic gas) = change in temperature =
= change in temperature = 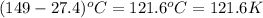
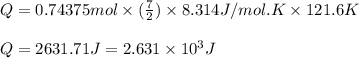
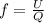
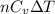
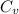 = specific heat capacity at constant pressure =
= specific heat capacity at constant pressure = 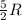 (For diatomic gas)
(For diatomic gas)