
Consider the following equation.
fe2o3(s) + 3h2(g) → 2fe(s) + 3h2o(g)
h = 98.8 kj, and s = 141.5 j/k. is this reaction spontaneous or nonspontaneous at high and low temperatures?
a. spontaneous at high temperatures, non-spontaneous at low temperatures
b. non-spontaneous at high and low temperatures
c. spontaneous at low temperatures, non-spontaneous at high temperatures
d. spontaneous at high and low temperatures

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
Consider the following equation.
fe2o3(s) + 3h2(g) → 2fe(s) + 3h2o(g)
h = 98.8 kj, and s...
fe2o3(s) + 3h2(g) → 2fe(s) + 3h2o(g)
h = 98.8 kj, and s...
Questions



Mathematics, 06.02.2021 01:00


Mathematics, 06.02.2021 01:00

English, 06.02.2021 01:00



History, 06.02.2021 01:00


History, 06.02.2021 01:00

Mathematics, 06.02.2021 01:00

Mathematics, 06.02.2021 01:00

Biology, 06.02.2021 01:00

Mathematics, 06.02.2021 01:00


Mathematics, 06.02.2021 01:00

Mathematics, 06.02.2021 01:10


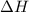 and the entropy change
and the entropy change 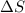 due to this reaction are positive. A chemical reaction will be spontaneous only if the change in its Gibbs Free Energy
due to this reaction are positive. A chemical reaction will be spontaneous only if the change in its Gibbs Free Energy 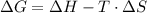 is negative.
is negative. 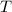 is the absolute temperature in degrees Kelvins.
is the absolute temperature in degrees Kelvins.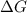 will initially be close to
will initially be close to 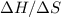 . The reaction will eventually become spontaneous.
. The reaction will eventually become spontaneous.

