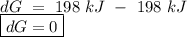1point
for a reaction, a h= 198 kj. for which value of ta sis the reaction
spontaneous?...

Chemistry, 21.07.2019 01:10 savyblue1724707
1point
for a reaction, a h= 198 kj. for which value of ta sis the reaction
spontaneous?
o a. -198 kj
o b. 198 kj
o c. oku
o d. 396 kj
submit

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
Questions


Chemistry, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00

Biology, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00





Mathematics, 26.02.2021 01:00


Mathematics, 26.02.2021 01:00

Business, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00





Spanish, 26.02.2021 01:00

 A positive ΔG represents a non-spontaneous reactionA negative ΔG value indicates a spontaneous reaction.
A positive ΔG represents a non-spontaneous reactionA negative ΔG value indicates a spontaneous reaction.