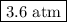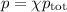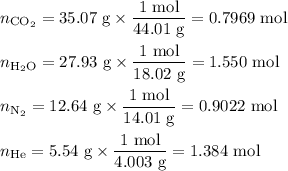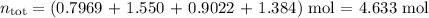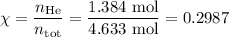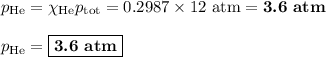Chemistry, 22.07.2019 05:10 tiwaribianca475
Amixture contains 35.07 grams of carbon dioxide, 27.93 grams of water vapor, 12.64 grams of nitrogen, and 5.54 grams of helium. the total pressure of the system is 12 atm, what is the partial pressure of the helium?
a. 0.88 atm
b. 0.82 atm
c. 0.073 atm
d. 0.068 atm

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Amixture contains 35.07 grams of carbon dioxide, 27.93 grams of water vapor, 12.64 grams of nitrogen...
Questions


Mathematics, 22.05.2020 00:01




Mathematics, 22.05.2020 00:01







Mathematics, 22.05.2020 00:01




Biology, 22.05.2020 00:01