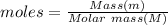Chemistry, 22.07.2019 16:10 MannyBanko1350
Methanol, ethanol, and n−propanol are three common alcohols. when 1.00 g of each of these alcohols is burned in air, heat is liberated as indicated. calculate the heats of combustion of these alcohols in kj/mol.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
Methanol, ethanol, and n−propanol are three common alcohols. when 1.00 g of each of these alcohols i...
Questions





Advanced Placement (AP), 15.12.2021 01:30







Mathematics, 15.12.2021 01:30






Mathematics, 15.12.2021 01:30

English, 15.12.2021 01:30

English, 15.12.2021 01:30