Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1


Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
Calculate the equilibrium constant for the following reaction: 2cl2(g) + 2h2o(g) ⇌ 4hcl(g) + o2(g)...
Questions


Biology, 05.10.2019 03:10



History, 05.10.2019 03:10

Physics, 05.10.2019 03:10














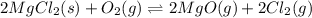

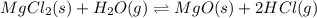

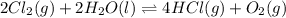
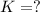
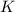 for the final reaction.
for the final reaction.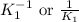 and multiplying the equation 2 by 2 that means we are writing the equilibrium constant as
and multiplying the equation 2 by 2 that means we are writing the equilibrium constant as 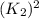 , we get the final equilibrium reaction and the expression of final equilibrium constant is:
, we get the final equilibrium reaction and the expression of final equilibrium constant is: