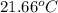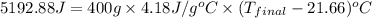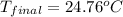Chemistry, 22.07.2019 23:20 autumnguidry7628
Aquantity of 2.00 × 102 ml of 0.461 m hcl is mixed with 2.00 × 102 ml of 0.231 m ba(oh)2 in a constant-pressure calorimeter of negligible heat capacity. the initial temperature of the hcl and ba(oh)2 solutions is the same at 21.66°c. for the process below, the heat of neutralization is −56.2 kj/mol. what is the final temperature of the mixed solutions? h+(aq) + oh−(aq) → h2o(l)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 05:00
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
Aquantity of 2.00 × 102 ml of 0.461 m hcl is mixed with 2.00 × 102 ml of 0.231 m ba(oh)2 in a consta...
Questions

Mathematics, 10.03.2021 22:20




History, 10.03.2021 22:20

Mathematics, 10.03.2021 22:20

Biology, 10.03.2021 22:20

Arts, 10.03.2021 22:20

Mathematics, 10.03.2021 22:20

Computers and Technology, 10.03.2021 22:20


World Languages, 10.03.2021 22:20

Chemistry, 10.03.2021 22:20


Social Studies, 10.03.2021 22:20




Chemistry, 10.03.2021 22:20

Biology, 10.03.2021 22:20

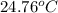
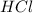 and
and 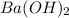 .
.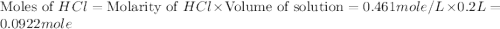
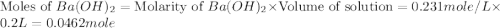
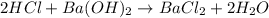
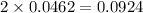 mole of HCl
mole of HCl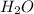
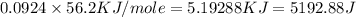
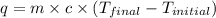
 = specific heat capacity =
= specific heat capacity = 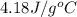
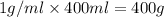
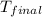 = final temperature = ?
= final temperature = ?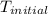 = initial temperature =
= initial temperature =