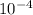Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2


Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
Calculate the molar solubility of ag2 cro4 in water. use 1.10 x 10-12 as the solubility product cons...
Questions


Biology, 04.01.2020 02:31


English, 04.01.2020 02:31




English, 04.01.2020 02:31

Mathematics, 04.01.2020 02:31

Social Studies, 04.01.2020 02:31



Biology, 04.01.2020 02:31







![s=\sqrt[3]{(1.10)(10^{-12})}=1.03](/tpl/images/0122/2625/96243.png) ×
×