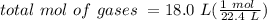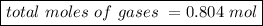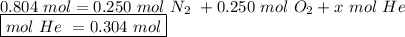Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3


Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
Avolume of 18.0 l contains a mixture of 0.250 mole n2 , 0.250 mole o2 , and an unknown quantity of h...
Questions

Geography, 28.05.2021 19:20

Mathematics, 28.05.2021 19:20



Mathematics, 28.05.2021 19:20

Mathematics, 28.05.2021 19:20

Mathematics, 28.05.2021 19:20

Mathematics, 28.05.2021 19:20


Mathematics, 28.05.2021 19:20

Mathematics, 28.05.2021 19:20

Advanced Placement (AP), 28.05.2021 19:20

Spanish, 28.05.2021 19:20

Mathematics, 28.05.2021 19:20


Chemistry, 28.05.2021 19:20



Geography, 28.05.2021 19:20

Advanced Placement (AP), 28.05.2021 19:20