

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 09:00
Spaghetti sauce can be high in sodium. what is a good guideline for mg of sodium per half cup serving? a. less than 1 mg b. less than 800 mg c. less than 700 mg d. less than 400 mg
Answers: 2
You know the right answer?
Where the oxygen comes from the air (21% o2 and 79% n2). if oxygen is fed from air in excess of the...
Questions



Mathematics, 21.05.2020 03:04

History, 21.05.2020 03:04

Geography, 21.05.2020 03:04



Mathematics, 21.05.2020 03:04

Biology, 21.05.2020 03:04


Geography, 21.05.2020 03:04

Mathematics, 21.05.2020 03:04


Mathematics, 21.05.2020 03:04




Mathematics, 21.05.2020 03:04

Mathematics, 21.05.2020 03:04

English, 21.05.2020 03:04

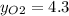 %
%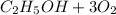 →
→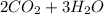
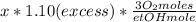
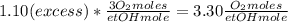
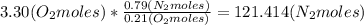
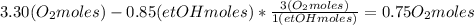
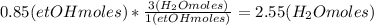
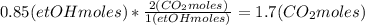
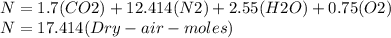
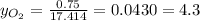 %
%

