
Chemistry, 23.07.2019 06:20 rainbowboy9231
Agaseous compound is 78.14 percent boron and 21.86 percent hydrogen. at 27°c, 74.3 ml of the gas exerted a pressure of 1.12 atm. if the mass of the gas was 0.0934 g, what is its molecular formula?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Estructura 7.2 completar repaso 1 - ¿lógico o ilógico? 1 - ¿lógico o ilógico? listen and indicate whether each question and response is lógico or ilógico.
Answers: 3

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
Agaseous compound is 78.14 percent boron and 21.86 percent hydrogen. at 27°c, 74.3 ml of the gas exe...
Questions


Arts, 14.04.2021 22:40

Mathematics, 14.04.2021 22:40

Mathematics, 14.04.2021 22:40

Mathematics, 14.04.2021 22:40

Chemistry, 14.04.2021 22:40

English, 14.04.2021 22:40





Mathematics, 14.04.2021 22:40


Mathematics, 14.04.2021 22:40



English, 14.04.2021 22:40


Mathematics, 14.04.2021 22:40

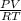
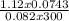
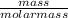
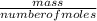
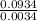
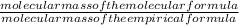
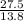 = 2
= 2


