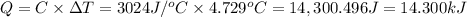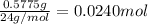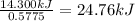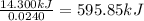A0.5775−g sample of solid magnesium is burned in a constant-volume bomb calorimeter that has a heat capacity of 3024 j/°c. the temperature increases by 4.729°c. (a) calculate the heat associated with the burning mg in kj/g. kj/g (b) calculate the heat associated with the burning of mg in kj/mol. kj/mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1
You know the right answer?
A0.5775−g sample of solid magnesium is burned in a constant-volume bomb calorimeter that has a heat...
Questions




English, 25.03.2020 05:06



Mathematics, 25.03.2020 05:07



Mathematics, 25.03.2020 05:07





Mathematics, 25.03.2020 05:07




Mathematics, 25.03.2020 05:07