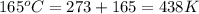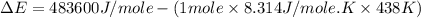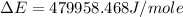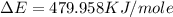Chemistry, 24.07.2019 11:20 brookeanne723
Consider the reaction 2h2o(g) → 2h2(g) + o2(g)δh = 483.6 kj/mol. if 2.0 moles of h2o(g) are converted to h2(g) and o2(g) against a pressure of 1.0 atm at 165°c, what is δu for this reaction?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
When the earth was formed and cooled, why did nickel and iron end up in the center of the earth while basalt and granite ended up in the outer layers
Answers: 3

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
Consider the reaction 2h2o(g) → 2h2(g) + o2(g)δh = 483.6 kj/mol. if 2.0 moles of h2o(g) are converte...
Questions


History, 11.09.2019 16:30

Physics, 11.09.2019 16:30

English, 11.09.2019 16:30

Mathematics, 11.09.2019 16:30

History, 11.09.2019 16:30







History, 11.09.2019 16:30





Biology, 11.09.2019 16:30

English, 11.09.2019 16:30

English, 11.09.2019 16:30

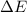 of the reaction is, 479.958 KJ/mole
of the reaction is, 479.958 KJ/mole
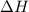 = enthalpy of the reaction = 483.6 KJ/mole = 483600 J/mole
= enthalpy of the reaction = 483.6 KJ/mole = 483600 J/mole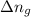 = change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole
= change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole