
You have two 466.0 ml aqueous solutions. solution a is a solution of silver nitrate, and solution b is a solution of potassium chromate. the masses of the solutes in each of the solutions are the same. when the solutions are added together, a blood-red precipitate forms. after the reaction has gone to completion, you dry the solid and find that it has a mass of 331.8 g. (a) calculate the concentration of the potassium ions in the original potassium chromate solution.(b) calculate the concentration of the chromate ions in the final solution

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
You have two 466.0 ml aqueous solutions. solution a is a solution of silver nitrate, and solution b...
Questions


Mathematics, 25.05.2020 18:57



Mathematics, 25.05.2020 18:57


Chemistry, 25.05.2020 18:57

Social Studies, 25.05.2020 18:57

Mathematics, 25.05.2020 18:57

Mathematics, 25.05.2020 18:57

English, 25.05.2020 18:57

Mathematics, 25.05.2020 18:57


Mathematics, 25.05.2020 18:57

English, 25.05.2020 18:57


Mathematics, 25.05.2020 18:57

Mathematics, 25.05.2020 18:57


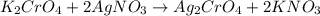
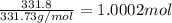
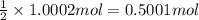 of silver nitrate.
of silver nitrate.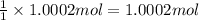 of potassium chromate
of potassium chromate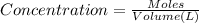
![[K^+]=\frac{2.0004 mol}{0.466 L}=4.2927 mol/L](/tpl/images/0127/0717/630d0.png)
![[CrO_4^{2+}]=\frac{1.0002 mol}{0.466 L+0.466L}=1.0731 mol/L](/tpl/images/0127/0717/e3c96.png)



