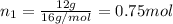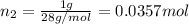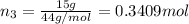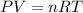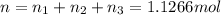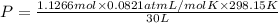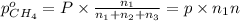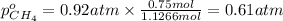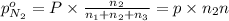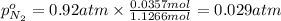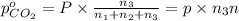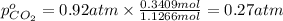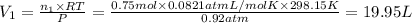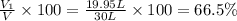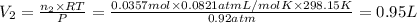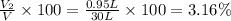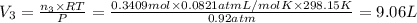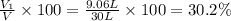Chemistry, 24.07.2019 16:10 jacksonhoyt8049
A30-liter volume of gas at 25°c contains 12 g of methane, 1 g of nitrogen, and 15 g of carbon dioxide. calculate (a) the moles of each gas present, (b) the partial pressure exerted by each gas, (c) the total pressure exerted by the mixture, and (d) the percentage by volume of each gas in the mixture. you may assume ideal gas behavior

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
A30-liter volume of gas at 25°c contains 12 g of methane, 1 g of nitrogen, and 15 g of carbon dioxid...
Questions

Mathematics, 25.06.2019 11:30


Mathematics, 25.06.2019 11:30

Business, 25.06.2019 11:30

English, 25.06.2019 11:30

History, 25.06.2019 11:30

Arts, 25.06.2019 11:30



Biology, 25.06.2019 11:30

English, 25.06.2019 11:30


History, 25.06.2019 11:30


Mathematics, 25.06.2019 11:30

Mathematics, 25.06.2019 11:30




Health, 25.06.2019 11:30