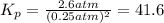Chemistry, 24.07.2019 17:10 devilrao6742
The reaction x 2 (g) m 2 x(g) occurs in a closed reaction vessel at constant volume and temperature. initially, the vessel contains only x 2 at a pressure of 1.55 atm. after the reaction reaches equilibrium, the total pressure is 2.85 atm. what is the value of the equilibrium constant, kp , for the reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
You know the right answer?
The reaction x 2 (g) m 2 x(g) occurs in a closed reaction vessel at constant volume and temperature....
Questions


Arts, 09.11.2020 18:40



Mathematics, 09.11.2020 18:40



Mathematics, 09.11.2020 18:40

Mathematics, 09.11.2020 18:40

Mathematics, 09.11.2020 18:40

Mathematics, 09.11.2020 18:40

History, 09.11.2020 18:40

Physics, 09.11.2020 18:40

Geography, 09.11.2020 18:40



History, 09.11.2020 18:40



Arts, 09.11.2020 18:40

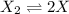
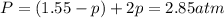
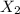 at equilibrium:
at equilibrium:![[p_{X_2}^o]=2p=2\time 1.3 atm=2.6 atm](/tpl/images/0128/0119/e4252.png)
![[p_{X}^{o}]=1.55 atm -1.3 atm = 0.25 atm](/tpl/images/0128/0119/f5d78.png)
![K_p=\frac{[p_{X_2}^o]}{[p_{X}^{o}]^2}](/tpl/images/0128/0119/aa6e2.png)