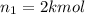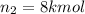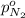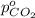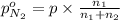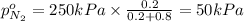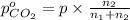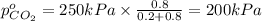Chemistry, 24.07.2019 19:10 holasoykawaii10
Arigid tank is divided into two equal volumes. one side contains 2 kmol of nitrogen n2 at 500 kpa while the other side contains 8 kmol of co2 at 200 kpa. the two sides are now connected and the gases are mixed and forming a homogeneous mixture at 250 kpa. find the partial pressure of the co2 in the final mixture.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
Arigid tank is divided into two equal volumes. one side contains 2 kmol of nitrogen n2 at 500 kpa wh...
Questions



Chemistry, 02.07.2019 08:00

Mathematics, 02.07.2019 08:00

Mathematics, 02.07.2019 08:00


Mathematics, 02.07.2019 08:00


Mathematics, 02.07.2019 08:00

Mathematics, 02.07.2019 08:00

Mathematics, 02.07.2019 08:00









English, 02.07.2019 08:00

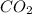 in the final mixture is 200 kPa.
in the final mixture is 200 kPa.