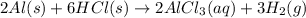Write an equation for the reaction of solid aluminum metal with hydrochloric acid (hydrogen monochloride) dissolved in water to form aluminum chloride dissolved in water and hydrogen gas. when balanced, what is the coefficient for the hydrochloric acid (hydrogen monochloride)?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1
You know the right answer?
Write an equation for the reaction of solid aluminum metal with hydrochloric acid (hydrogen monochlo...
Questions


Mathematics, 31.01.2020 23:59


History, 31.01.2020 23:59



History, 31.01.2020 23:59


Spanish, 31.01.2020 23:59



Mathematics, 31.01.2020 23:59


Physics, 31.01.2020 23:59


Mathematics, 31.01.2020 23:59



Mathematics, 31.01.2020 23:59