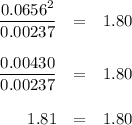Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80 at 250 ∘c a 0.197 mol sample of pcl5(g) is injected into an empty 2.90 l reaction vessel held at 250 ∘c. calculate the concentrations of pcl5(g) and pcl3(g) at equilibrium.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
You know the right answer?
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80...
Questions



History, 02.02.2020 19:56


Arts, 02.02.2020 19:56


Mathematics, 02.02.2020 19:56

History, 02.02.2020 19:56

Social Studies, 02.02.2020 19:56

History, 02.02.2020 19:56



Mathematics, 02.02.2020 19:56

Computers and Technology, 02.02.2020 19:56


Mathematics, 02.02.2020 19:56

Mathematics, 02.02.2020 19:56



Computers and Technology, 02.02.2020 19:56

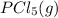 is 0.0655 M and
is 0.0655 M and 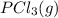 is 0.00240 M at equilibrium.
is 0.00240 M at equilibrium.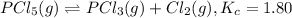
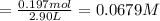

![[PCl_3]=x](/tpl/images/0128/9812/3c44a.png)
![[Cl_2] = x](/tpl/images/0128/9812/a7a23.png)
![=[PCl_5]= (0.0697- x)](/tpl/images/0128/9812/ead0f.png)
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}\\\\1.80=\frac{x\times x}{(0.0679-x)}\\\\x = 0.0655](/tpl/images/0128/9812/79ef0.png)
![=[PCl_5]= (0.0679- x) = (0.0679 -0.0655 )M=0.00240 M](/tpl/images/0128/9812/ac2f8.png)
![= [Cl_2] = x = 0.0655 M](/tpl/images/0128/9812/6faf5.png)
![\text{[PCl$_{5}$]} = \dfrac{\text{0.197 mol}}{\text{2.90 L}} = \text{0.067 93 mol/L}\\\\](/tpl/images/0128/9812/66356.png)
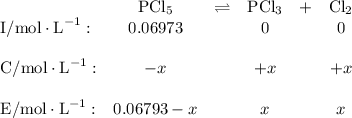
![K_{\text{c}} = \dfrac{\text{[PCl$_3$][Cl$_2$]}}{\text{[PCl$_5$]}} = \dfrac{x^{2}}{0.06793-x} = 1.80\\\\\begin{array}{rcl}\\x^{2}& = & 1.80(0.06793 - x)\\x^{2& = & 0.1223 - 1.80x\\x^{2} + 1.80x - 0.1223& = & 0\\x & = & \mathbf{0.0656}\\\end{array}](/tpl/images/0128/9812/cf1bb.png)