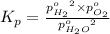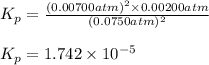Chemistry, 25.07.2019 01:20 jaymee2904p88tgh
The elementary reaction 2h2o(g)↽−−⇀2h2(g)+o2(g) proceeds at a certain temperature until the partial pressures of h2o, h2, and o2 reach 0.0750 atm, 0.00700 atm, and 0.00200 atm, respectively. what is the value of the equilibrium constant at this temperature?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

You know the right answer?
The elementary reaction 2h2o(g)↽−−⇀2h2(g)+o2(g) proceeds at a certain temperature until the partial...
Questions

Mathematics, 29.09.2020 22:01





Social Studies, 29.09.2020 22:01

Mathematics, 29.09.2020 22:01

History, 29.09.2020 22:01

Mathematics, 29.09.2020 22:01


Mathematics, 29.09.2020 22:01

History, 29.09.2020 22:01

Mathematics, 29.09.2020 22:01

English, 29.09.2020 22:01

History, 29.09.2020 22:01





 is the value of the equilibrium constant at this temperature.
is the value of the equilibrium constant at this temperature.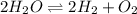
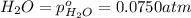
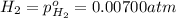
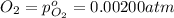
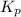 for the given chemical equation is:
for the given chemical equation is: