
Chemistry, 25.07.2019 03:30 Alphonse8472
Consider the following reaction. how many grams of water are required to form 75.9 g of hno3? assume that there is excess no2 present. the molar masses are as follows: h2o = 18.02 g/mol, hno3 = 63.02 g/mol. no2(g) + h2o(l) → hno3(aq) + no(g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

You know the right answer?
Consider the following reaction. how many grams of water are required to form 75.9 g of hno3? assum...
Questions



English, 15.04.2021 23:30

Mathematics, 15.04.2021 23:30

Chemistry, 15.04.2021 23:30



Mathematics, 15.04.2021 23:30

Mathematics, 15.04.2021 23:30



Mathematics, 15.04.2021 23:30


Mathematics, 15.04.2021 23:30

Mathematics, 15.04.2021 23:30




Social Studies, 15.04.2021 23:30

Mathematics, 15.04.2021 23:30

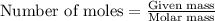 ......(1)
......(1)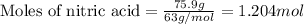
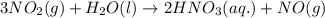
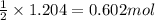 of water.
of water.


