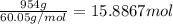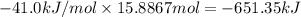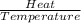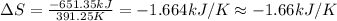Chemistry, 25.07.2019 04:20 thawkins79
The heat of vaporization δhv of acetic acid hch3co2 is 41.0 /kjmol. calculate the change in entropy δs when 954.g of acetic acid condenses at 118.1°c. be sure your answer contains a unit symbol. round your answer to 3 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1


You know the right answer?
The heat of vaporization δhv of acetic acid hch3co2 is 41.0 /kjmol. calculate the change in entropy...
Questions



Biology, 21.10.2020 19:01

Mathematics, 21.10.2020 19:01

Mathematics, 21.10.2020 19:01

Arts, 21.10.2020 19:01

Chemistry, 21.10.2020 19:01


History, 21.10.2020 19:01

Geography, 21.10.2020 19:01

History, 21.10.2020 19:01

Mathematics, 21.10.2020 19:01

World Languages, 21.10.2020 19:01


English, 21.10.2020 19:01




Mathematics, 21.10.2020 19:01