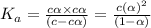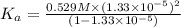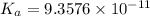Chemistry, 25.07.2019 06:20 swansondonovanp66got
In the laboratory a student measures the percent ionization of a 0.529 m solution of phenol (a weak acid) , c6h5oh, to be 1.33×10-3 %. calculate value of ka from this experimental data.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3


Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
In the laboratory a student measures the percent ionization of a 0.529 m solution of phenol (a weak...
Questions



Mathematics, 27.03.2020 03:07

Mathematics, 27.03.2020 03:07



Mathematics, 27.03.2020 03:07




Mathematics, 27.03.2020 03:07


Mathematics, 27.03.2020 03:07

Mathematics, 27.03.2020 03:07



Chemistry, 27.03.2020 03:07



 .
.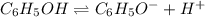
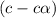
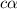
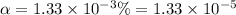
![K_a=\frac{[C_6H_5O^-][H^+]}{[C_6H_5OH]}](/tpl/images/0130/1052/3ceac.png)