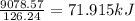Chemistry, 25.07.2019 20:20 NatalieAllen11
Enter your answer in the provided box. pentaborane−9 (b5h9) is a colorless, highly reactive liquid that will burst into flames when exposed to oxygen. the reaction is 2b5h9(l) 12o2(g) → 5b2o3(s) 9h2o(l) calculate the kilojoules of heat released per gram of the compound reacted with oxygen. the standard enthalpy of formations of b5h9(l), b2o3(s), and h2o(l) are 73.2, −1271.94, and −285.83 kj/mol, respectively.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1
You know the right answer?
Enter your answer in the provided box. pentaborane−9 (b5h9) is a colorless, highly reactive liquid t...
Questions


History, 17.07.2019 16:10


Social Studies, 17.07.2019 16:10

Mathematics, 17.07.2019 16:10


Biology, 17.07.2019 16:10

Physics, 17.07.2019 16:10

Computers and Technology, 17.07.2019 16:10

English, 17.07.2019 16:10

Business, 17.07.2019 16:10

History, 17.07.2019 16:10


Mathematics, 17.07.2019 16:10

English, 17.07.2019 16:10

English, 17.07.2019 16:10


History, 17.07.2019 16:10

Mathematics, 17.07.2019 16:10

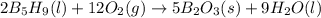
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0132/3420/76c37.png)
![\Delta H=[(n_{H_2O}\times \Delta H_{H_2O})+(n_{B_2O_3}\times \Delta H_{B_2O_3})]-[(n_{B_5H_9}\times \Delta H_{B_5H_9})+(n_{O_2}\times \Delta H_{O_2})]](/tpl/images/0132/3420/48532.png)
![\Delta H=[(9\times -285.83)+(5\times -1271.94)]-[(2\times 73.2)+(12\times 0)]\\\\\Delta H=-9078.57kJ](/tpl/images/0132/3420/9a745.png)
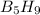 has 63.12 grams of mass
has 63.12 grams of mass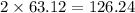 grams of mass
grams of mass