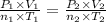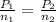Chemistry, 25.07.2019 21:10 heavendl13
Aclosed 1l chamber of gas undergoes the following reaction at 400 °c and 20,000 kpa: 2h2(g)+ o2(g) --> 2h2o(g) assuming 2 mols of hydrogen gas, 1 mol of oxygen gas, a constant temperature, and no product at start, what is the resulting pressure after the reaction occurs?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
Aclosed 1l chamber of gas undergoes the following reaction at 400 °c and 20,000 kpa: 2h2(g)+ o2(g)...
Questions


Mathematics, 11.02.2021 20:00



Mathematics, 11.02.2021 20:00


History, 11.02.2021 20:00



Social Studies, 11.02.2021 20:00


History, 11.02.2021 20:00



Mathematics, 11.02.2021 20:00

Mathematics, 11.02.2021 20:00

Spanish, 11.02.2021 20:00

Mathematics, 11.02.2021 20:00


Chemistry, 11.02.2021 20:00