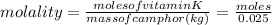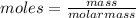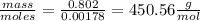Chemistry, 25.07.2019 21:20 Natasha019
Vitamin k is involved in normal blood clotting. when 0.802 g of vitamin k is dissolved in 25.0 g of camphor, the freezing point of the solution is lowered by 2.69 °c. the freezing point and kf constant for camphor can be found here. calculate the molar mass of vitamin k.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1

Chemistry, 23.06.2019 16:30
Amodel of an atom is shown below. which element is represented by this model of an atom? boron, carbon, neon, or sodium?
Answers: 1
You know the right answer?
Vitamin k is involved in normal blood clotting. when 0.802 g of vitamin k is dissolved in 25.0 g of...
Questions




Biology, 30.05.2021 16:00








Mathematics, 30.05.2021 16:00



Physics, 30.05.2021 16:00




Arts, 30.05.2021 16:10